Vertiflex® Interspinous Spacer in Valencia, CA and Lancaster, CA

Dr. Thomas Nasser is a leading Pain Management and Doctor of Physical Medicine and Rehabilitation. Dr. Nasser is also the Medical Director at Palmdale Regional Medical Center Acute Rehabilitation Institute, Embassy Surgery Center, and Antelope Valley Care Center. Dr. Thomas Nasser has been treating lumbar spinal stenosis (LLS) in the Antelope Valley since 2007. LLS is a condition in which the lower spinal canal narrows and compresses the spinal nerves in the lower back.
Dr. Nasser treats “the whole person” not just the symptoms. By evaluating the impact of a condition on the person as a whole (medically, socially, emotionally and vocationally), he helps his patients understand and take control of their health.
One procedure that has high clinical success in helping patients suffering from LLS in the Antelope Valley is The Vertiflex® interspinous spacer. This procedure is a minimally invasive approach that allows the team at AVORS Medical Group provide relief to our patients struggling with LSS.
For patients with lumbar spinal stenosis, there are not many treatment options that bridge the gap between conservative care and invasive surgery. However, the Vertiflex® interspinous spacer is a minimally invasive procedure that offers patients with LSS many distinct advantages.
The spine undergoes many degenerative changes as a natural result of aging. However, some symptoms may actually be associated with a condition called lumbar spinal stenosis (LSS). As it progresses, patients often experience unpleasant symptoms such as leg pain, numbness and cramping and decreased endurance when standing or walking.
Once you’ve received an LSS diagnosis, most doctors opt for conservative measures first and foremost. If this fails to provide relief from adverse symptoms, there aren’t many non-surgical options — that is, until now.
English Vertiflex
Spanish Vertiflex
If you believe you might be a candidate for the Vertiflex Procedure, ask yourself the following questions:
- Do you experience pain or weakness in your back, buttocks, or legs when you stand or walk?
2. Do you use a cane, walker, or shopping cart to move around more comfortably?
3. Do you frequently need to lean or bend forward, or sit down to find relief?
4. Do you want to find an alternative option to lumbar surgery?
If you answered yes to any of the four questions above, you may be a candidate for the Vertiflex Procedure.
Call AVORS Medical Group to schedule your consultation at 661-726-5005.
Patient Testimonials
Clinical References
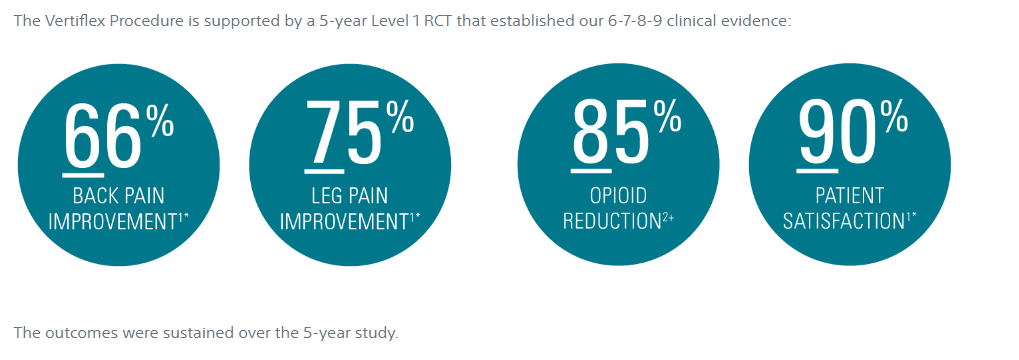
1. Nunley PD, Patel VV, Orndorff DG, Lavelle WF, Block JE, Geisler FH. Five-year durability of stand-alone interspinous process decompression for lumbar spinal stenosis. Clin Interv Aging. 2017;12:1409-1417. (N = 88)
2. Nunley PD, Deer TR, Benyamin RM, Staats PS, Block JE. Interspinous process decompression is associated with a reduction in opioid analgesia in patients with lumbar spinal stenosis. J Pain Res. 2018;11:2943-2948. (N = 107)
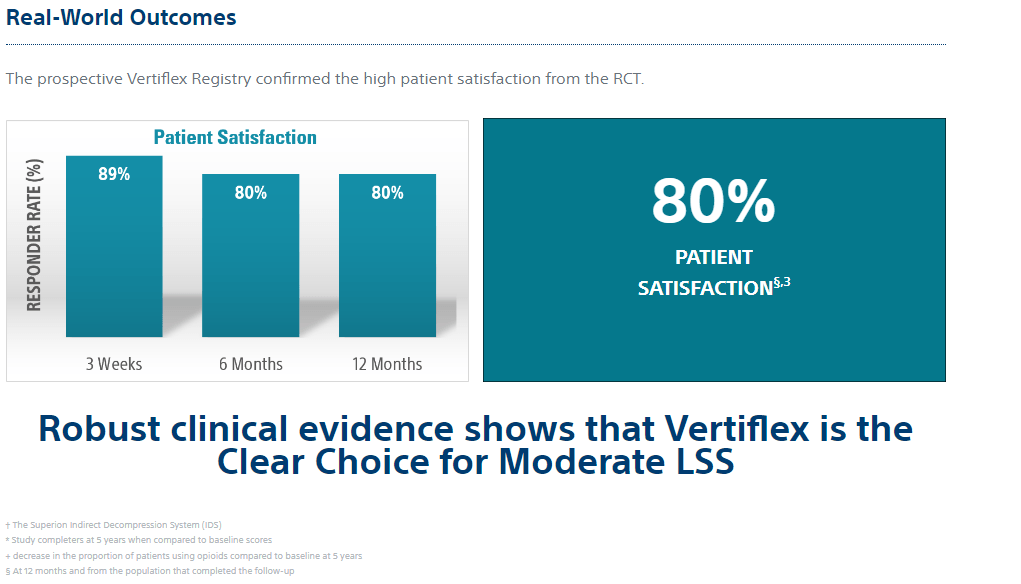
3. Tekmyster G, Sayed D, Cairns KD, Raso LJ, Kim C, Block JE. Interspinous Process Decompression With The Superion Spacer For Lumbar Spinal Stenosis: Real-World Experience From A Device Registry. Med Devices (Auckl). 2019;12:423-427. (N = 368)




